An astonishing number of proteins are processed and travel through the endoplasmic reticulum in our cells. When there is a malfunction in this process, proteins can clump together. If these aggregates aren’t cleaned up, the result can be any number of debilitating diseases.
The primary focus at Jace Biomedical (JBI) is to alleviate the burden caused by these proteotoxic diseases. By precisely increasing the degradation of only the proteins in the endoplasmic reticulum, we resolve the aggregation of toxic proteins while avoiding side-effects that would result from upregulating protein breakdown in the whole cell.
The Problem: Proteotoxic Diseases
The endoplasmic reticulum (ER) processes proteins in our cells that are membrane bound, destined for organelles, secreted and/or post translationally modified. In total, approximately 30% of all human proteins move through the ER. With such a large flux of proteins moving through one space, it’s no surprise that sometimes things go wrong: proteins harbor deleterious mutations, protein production is increased beyond the ER’s capacity, or degradation is slowed to sub-optimal rates. All of these possibilities have the potential to result in the destructive build-up of protein.
Excessive aggregation of protein can cripple the cell’s ability to carry out basic functions and can even lead to cell death. Furthermore, these aggregates can be secreted outside the cell, causing further damage. This kind of protein-aggregation has been linked to a variety of ailments including neurodegeneration, cardiovascular and musculoskeletal diseases, metabolic disorders, cancers and susceptibility to a variety of infections. Alzheimer’s Disease is one infamous indication linked to ER-based protein aggregation that impacts a huge population, with 500,000 people a year receiving an AD diagnosis annually in the US alone. At the same time, a notable number of diseases, including many rare diseases, have been identified that are the result of the mutation and/or build up of a single protein in the ER.

Our Solution: Regulate Autophagy in the ER
Cells degrade misfolded, aggregated or unnecessary proteins through the process of autophagy. Autophagy specific to the ER is known as reticulophagy. Reticulophagy is regulated through quality control that is signaled through a recently discovered mechanism of protein acetylation in the ER. Our lead program uses small molecules to intervene at this quality control checkpoint, turning the dial to upregulate reticulophagy and breakdown toxic protein aggregates residing and being formed specifically in the ER.
JBI is building a therapeutic pipeline. Initially this approach was developed as an intervention for the incredibly burdensome Alzheimer’s disease (AD). Since then, research efforts have identified multiple diseases that result from protein aggregation through the secretory pathway. Alpha-1 antitrypsin deficiency (A1ATD) associated liver disease is one promising indication, with a largely agreed upon disease mechanism. In A1ATD liver disease, mutated AAT protein misfolds and aggregates in the ER. The aggregates build up, cause ER stress and cell death that leads to liver fibrosis and potential cirrhosis. JBI’s proposed therapeutic intervention would intervene in this disease cascade via a small molecule that inhibits acetylation of the protein ATG9a in order to induce reticulophagy, break down misfolded and aggregated protein and prevent subsequent cellular injury and fibrosis.
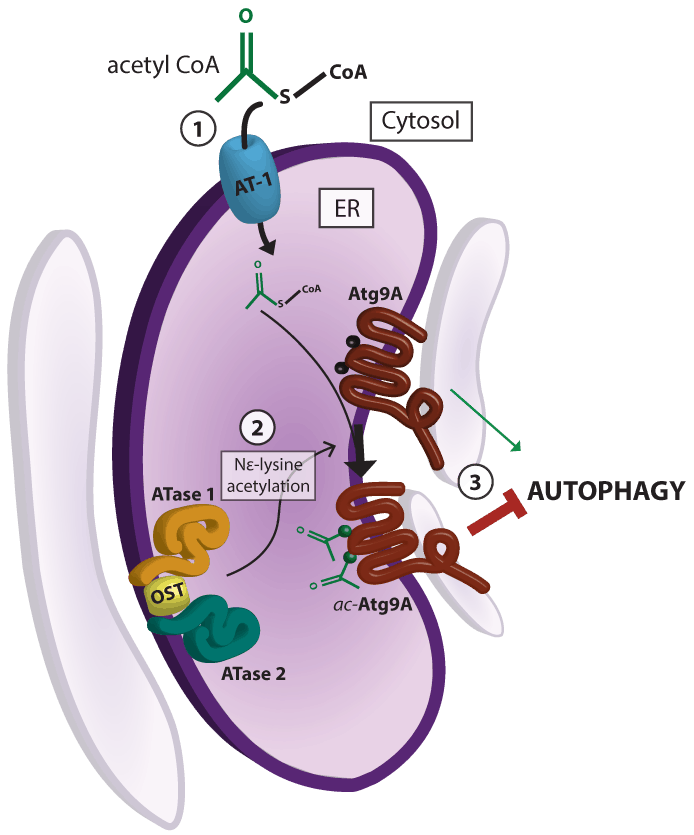
(Photo Above) Protein degradation is inhibited by acetylation of specific proteins in the ER:
Quality control mechanisms in the ER downregulate autophagy through the acetylation of Atg9A by two recently discovered acetyltransferases (ATase1 and ATase2). Our approach uses small molecules to target proteins in this pathway and release inhibition of autophagy, leading to increased degradation of toxic protein aggregates.
The strength of our approach
Targeted autophagy induction
Many current autophagy inducing therapeutics in development upregulate autophagy globally within the cell, which has the potential to cause a variety of off-target effects. Our mechanistic approach selectively upregulates autophagy in the ER, right where pathogenic aggregates are forming, reducing the risk of problematic side effects and off-target toxicity.
One therapeutic, many indications
Aggregation of ER-proteins has been linked to a variety of diseases that impact a range of populations. Once validated, our approach has the potential to be optimized to treat a variety of diseases, from widespread diseases like AD, cancer and infections, to a number of rare and orphan diseases.
Combination approach potential
A number of developing and approved therapeutics targeting protein-aggregation diseases work through clearing existing aggregates. We believe our drug mechanism would be a powerful combination with these therapies to inhibit the formation of new aggregates.
Identifiable patient populations
Many indications caused by ER protein aggregation are linked to specific genetic mutations. Simple screening for these mutations has the potential to quickly identify the patients most likely to be helped by this therapeutic approach.
Backed by Science
Want to learn more? Here’s a good place to start
ATase inhibition rescues age-associated proteotoxicity of the secretory pathway
Nature, Communications Biology (2022)“The removal of toxic protein aggregates through reticulophagy is a particularly important aspect of the proteostatic functions of ER acetylation...our study demonstrates that inhibition of the ATases restores proteostatic functions within the ER and can rescue the disease phenotype …”
Nε-lysine acetylation in the endoplasmic reticulum – a novel cellular mechanism that regulates proteostasis and autophagy
Journal of Cell Science (2018)“By using a combination of cell- and animal-based studies, we discovered that inhibition of the ER acetylation machinery can stimulate autophagy-mediated disposal of toxic protein aggregates that form within the ER and secretory pathway but not those that form in the cytosol.”
Improved proteostasis in the secretory pathway rescues Alzheimer’s disease in the mouse
Brain (2016)“… the above results indicate that biochemical inhibition of ATase1/ATase2 can rescue the Alzheimer’s disease-like phenotype displayed by APP695/swe mice in the absence of evident toxicity.”
Protein Aggregation in the ER: Calm behind the Storm
Cells (2021)“Boosting the clearance of hepatic Z-AAT aggregates by autophagy inducers or gene overexpression can efficiently alleviate liver damages in pre-clinical animal models of AATD.”
ATase inhibition rescues age-associated proteotoxicity of the secretory pathway
Nature, Communications Biology (2022)“The removal of toxic protein aggregates through reticulophagy is a particularly important aspect of the proteostatic functions of ER acetylation...our study demonstrates that inhibition of the ATases restores proteostatic functions within the ER and can rescue the disease phenotype …”
Nε-lysine acetylation in the endoplasmic reticulum – a novel cellular mechanism that regulates proteostasis and autophagy
Journal of Cell Science (2018)“By using a combination of cell- and animal-based studies, we discovered that inhibition of the ER acetylation machinery can stimulate autophagy-mediated disposal of toxic protein aggregates that form within the ER and secretory pathway but not those that form in the cytosol.”
Improved proteostasis in the secretory pathway rescues Alzheimer’s disease in the mouse
Brain (2016)“… the above results indicate that biochemical inhibition of ATase1/ATase2 can rescue the Alzheimer’s disease-like phenotype displayed by APP695/swe mice in the absence of evident toxicity.”
Protein Aggregation in the ER: Calm behind the Storm
Cells (2021)“Boosting the clearance of hepatic Z-AAT aggregates by autophagy inducers or gene overexpression can efficiently alleviate liver damages in pre-clinical animal models of AATD.”
About Jace Biomedical, Inc.
Origins
JBI is a small but growing pharmaceutical company founded by two veterans of the industry, Carl Horn and Deborah Milkowski, PhD. Our focus is developing novel therapies for proteotoxic diseases of the secretory pathway that have no or limited therapeutic options.
After working together for several years at TAP Pharmaceuticals (the JV between Takeda and Abbott Laboratories) strategically building its pipeline, Mr. Horn and Dr. Milkowski once again joined forces in recent years. They began collaborative discussions with Dr. Luigi Puglielli from the University of Wisconsin-Madison to explore the potential of translating his novel research into a groundbreaking treatment for millions of patients afflicted with Alzheimer’s disease (AD).
Our Future
Since it’s founding, the focus of JBI has expanded substantially. As research has progressed, a variety of diseases resulting from protein aggregation in the secretory pathway have come to light. We continue to explore how JBI can intervene in these diseases.
We are excited about our future and believe that our technology can make a genuine impact in the lives of many. We’re poised to move forward and are anxious to meet our goals. Our immediate priorities include compound optimization and pre-IND studies with the longer-term goals of clinical trials and moving into patients.
If you are interested in learning more about ways you can partner with us to reach these milestones, we’d love to hear from you.
Carl Horn, Founder
Carl Horn brings over 25 years of pharmaceutical, diagnostics and biotech experience to JBI. Prior to JBI, he served as Senior Vice President, Corporate and Business Affairs at Therapeutic Proteins International, LLC (TPI). At TPI, Carl successfully guided the company through its acquisition by Amneal Enterprises, LLC. Prior to TPI, Carl was General Manager Global Export Business for Takeda. Prior to Takeda, Carl led the business development function at TAP Pharmaceuticals for seven years. Prior to TAP, Carl worked at Abbott Laboratories in various marketing and strategic planning leadership positions.
Deborah Milkowski, Phd, Founder
Dr. Milkowski has more than 13 years of industry experience in senior roles at TAP Pharmaceuticals where she was the Director of the Pharmacology and Technical Assessment organizations. Dr. Milkowski oversaw the pharmacology sections of 10 IND and 4 NDA submissions, including approvals for Lupron Depot® and Uloric®. She was responsible for the safety and efficacy pharmacology for all development compounds as well as for the technical assessments of all in-licensed technology. Dr. Milkowski received her PhD in Molecular Biology and Biochemistry from Northwestern University Medical School.
Contact us
Have questions? Want to partner with us?
We’d love to hear from you.